Introduction of DMFC Fuel Cell
- The DMFC is similar to polymer electrolyte membrane ( PEM ) cell in that both fuel cells use a polymer membrane as the electrolyte.
- However, the anode of DMFC draws hydrogen from the liquid methanol, eliminating the need of fuel reformer.
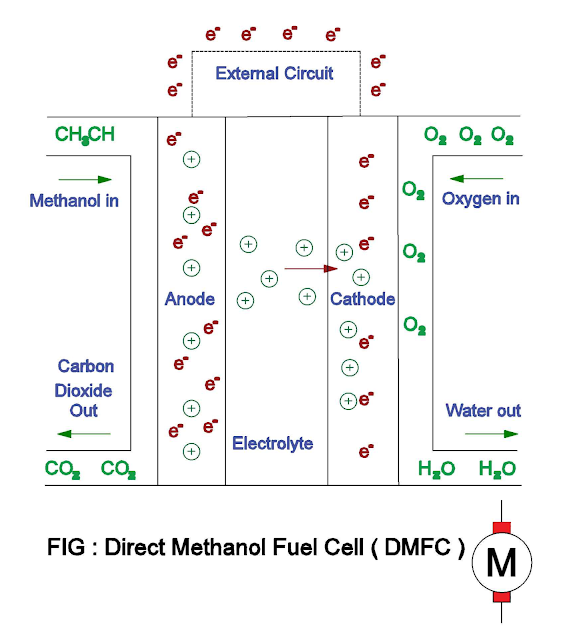
Direct Methanol Fuel Cells ( DMFC )
- The direct methanol fuel cells are powered by pure methanol which is mixed with water and fed directly to the fuel cell anode.
- The methanol has higher energy density as compared to hydrogen therefore it has not any fuel storage problem.
- The density of methanol is less than gasoline or diesel fuel. It is easy to transport and supply to public in the liquid form.
The methanol is directly oxidized to
carbon dioxide at the anode. At the cathode, oxygen gas combines with the
protons and electrons, reduces to water.
- Anode : CH3CH + H2O → CO2 + 6H+ + 6e-
- Cathode : 3/2O2 + 6H+ + 6e- → 3H2O
- Overall chemical reaction : CH3CH + 3/2O2 → 2H2O + CO2
|
You may also like to read these articles : |
FCT - DMFC
DMFC: Advantages
- Light weight
- Easy to transport
- Methanol is inexpensive, it can be supplied to fuel cell from liquid reservoir.
- No fuel storage problem
- Higher density as compared to hydrogen
- Supply to public through current infrastructure ( no additional cost )
DMFC: Disadvantages / Limitations
- Methanol cross over from anode to cathode across membrane separator.
Methanol crossover
- Excess methanol is supplied to anode side of the membrane electrode assembly.
- It is desirable that most of methanol to diffuse into anode and react.
- This phenomenon is called as methanol crossover in which some of methanol diffuses through polymer electrolyte membrane from anode to cathode.
- The dilute methanol is supplied to the anode electrode which diffuses into anode and partially reacts.
- The remaining methanol leaves electrodes and moves to cathode which result in reduce fuel efficiency and reduce cathode performance.
- The potential efficiency of DMFC for operating cell of 0.5 V is about 40% at temperature of 40 – 90 0C. Higher efficiency is achieved at higher temperatures.
DMFC: Application
- As a portable fuel cell for laptop computers or cell phone
- Defense and military
- Mobile homes and marine application








No comments:
Post a Comment